Merit Sensor AP Series in Pharmaceutical and Medical Applications
In the field of medical science, pressure is an indispensable physiological parameter. In a range of environments and applications involving both liquids and gases, precise measurement is essential for diagnostic accuracy and therapeutic efficacy.1 Merit Sensor Systems, a leader in MEMS piezoresistive pressure sensing, provides high-performing solutions for a range of industries.2 Among their innovative products, the AP Series pressure sensors are specially designed for pharmaceutical and medical applications.
In this article, we examine two configurations of the AP Series pressure sensor:
- AP-Series with polycarb port, or ferrule port for direct backside media contact
- AP-Series with gel-port plus dielectric gel for enhanced media biocompatibility
This is followed by an exploration into their application in various medical and pharmaceutical processes, including the integration of these sensors in Merit Medical’s Blue Diamond™ Digital Inflation Device.
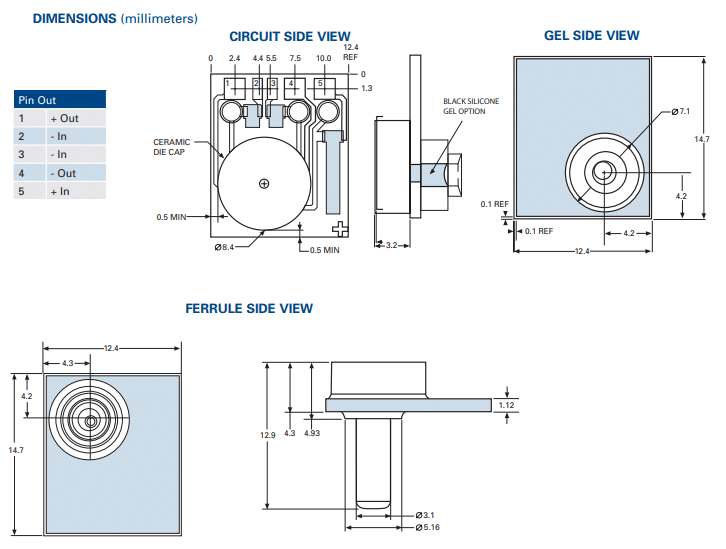
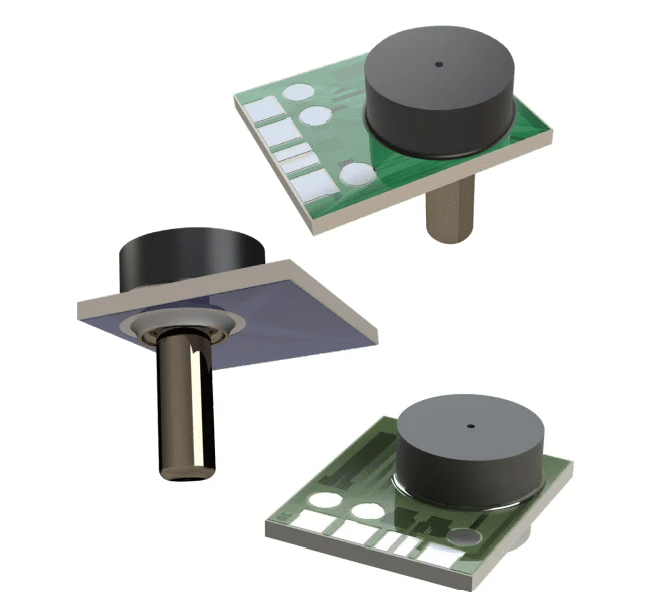
The AP-Series: Examining the Sensor’s Dual Configurations
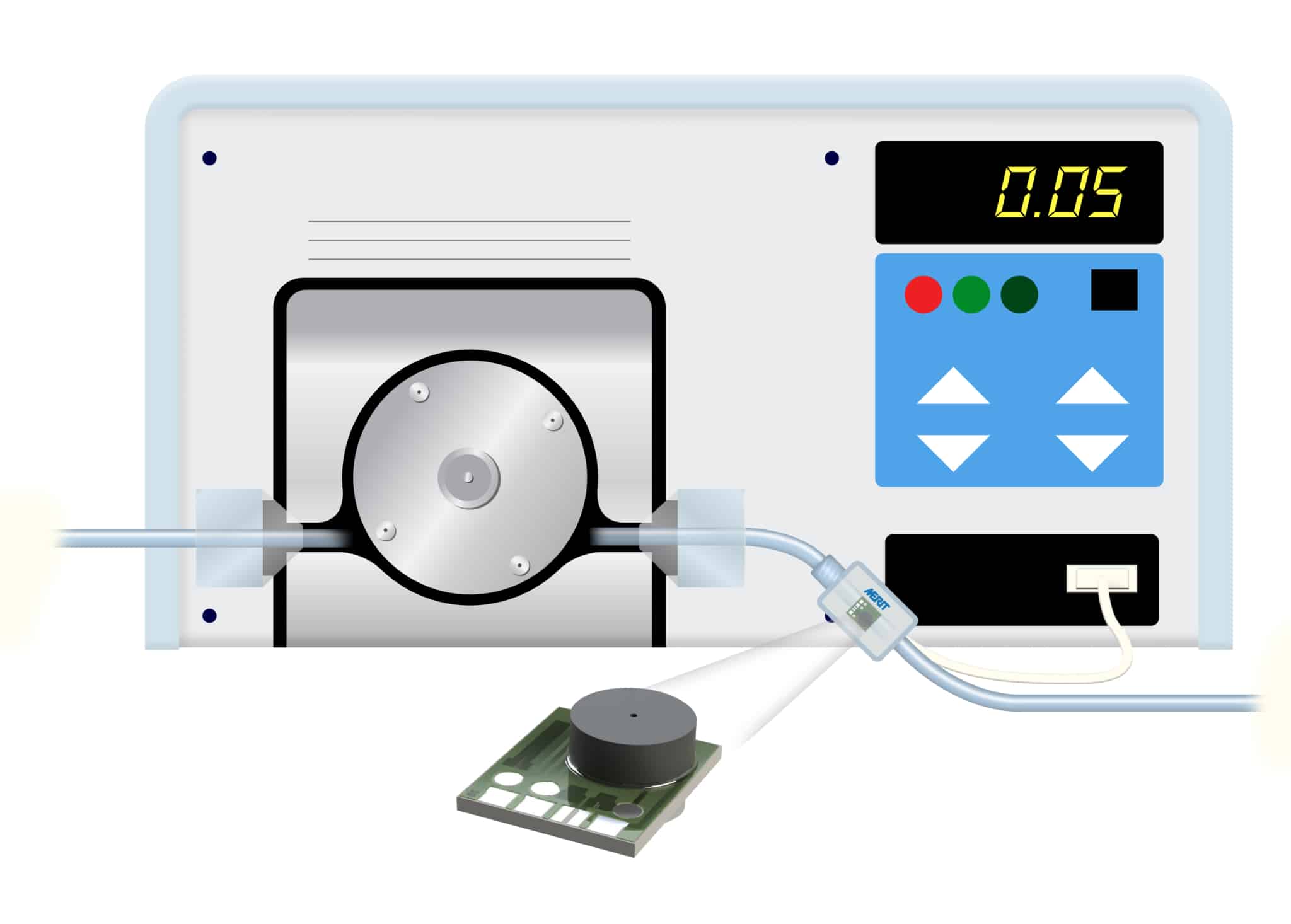
Utilizing a piezoresistive Wheatstone bridge with a chemically etched silicon diaphragm, both configurations of the AP-Series pressure sensor are engineered to provide precise pressure measurements in both static and dynamic environments.3 Each sensor, capable of measuring both liquids and gases, ensures cost-effectiveness without compromising on functionality.
Key Features
- Wide Pressure Range: The AP-Series sensors cover several apressure ranges from 0 to 1000 psi (0 to 70 bar) accommodating a variety of medical and pharmaceutical applications, from vacuum aspiration applications, to low pressure blood pressure, up to high pressure injection and pump control applications. 4
- High Accuracy: The sensors offer a trimmed output signal with minimal offset for easy amplification with an operational amplifier. This is achieved by calibrating the sensors using laser-trimmed resistors.4
- Robustness: Designed to withstand high burst pressures, these sensors are reliable even in challenging environments. Their compact geometry and durability make them suitable for integration into a multitude of medical devices. The design is suitable for single-use disposable applications, as well as long term applications.
- Sterilization Compatibility: These sensors are compatible with sterilization processes, including ETO, Gamma-ray, and autoclave, ensuring they meet the rigorous standards required in medical applications.
- Easy Integration: With options for flexible or cable interfaces, the AP-Series sensors can be seamlessly integrated into existing systems.
- High Resolution and Frequency: These sensors deliver a pure analog signal to allow exceptional performance in high-resolution and high-frequency applications.3
- Reliable Through Backside: Both configurations ensure that the active side of the sensing element (wirebonds and conductor traces) remains protected from direct contact with the media, enhancing the sensor’s longevity and reliability.
Applications
The AP-Series sensors, which have a polycarb and ferrule port as well as direct media contact on the backside, are suited for measuring liquid and gas pressure and flow in medical and pharmaceutical equipment. Typical applications include:
- Mixing Machines: Guaranteeing accurate pressure control during the mixing of pharmaceuticals.
- Analysis Machines: Precise pressure monitoring in laboratory equipment for fluid analysis.
- Fluid Treatment and Processing: This is used in systems that require precise pressure regulation to ensure the integrity of the fluid being processed.
- Aseptic Liquid Processing: Key when it comes to maintaining sterility and preventing contamination during the production of pharmaceuticals.
The AP-Series pressure sensors feature enhanced media compatibility with gel-port and dielectric gel, reducing wetted materials and making them ideal for challenging environments in pharmaceutical and medical applications, including:
- Bio-Decontamination Equipment: Monitoring pressure in systems that are created to sterilize equipment and facilities.
- Bioreactor Monitoring, Chromatography, and Flow Filtration: Ensuring reliable pressure measurements in critical biocompatibility processes.
- Laboratory Equipment: Providing precise pressure data in equipment used for sophisticated chemical analyses and experiments.
Integration in the Blue Diamond™ Digital Inflation Device
A notable application of the AP-Series pressure sensors is in Merit Medical’s Blue Diamond™ Digital Inflation Device and Fluid Dispensing Syringe. This device is designed to inflate and deflate balloon angioplasty catheters and other interventional devices. It can also be used to accurately measure the pressure and duration of inflation while simultaneously dispensing fluids into the body and monitoring pressure levels.5
Features of the Blue Diamond™ Digital Inflation Device
- Integral Pressure Transducer: The AP-Series sensors integrated into this device provide exact pressure measurements essential for the safe and effective performance of angioplasty procedures.5
- Microcomputer and Back-Lit LCD: These features facilitate precise control and easy monitoring of the inflation process in low and no-light situations.5,6
- Threaded Plunger Assembly and High-Pressure Extension Tube: Combined with Merit Sensor’s robust pressure sensors, these components ensure the device can generate and monitor positive and negative pressures over a range of -0.4 to +30 ATM/BAR (-6 to 441 psi).5
- Data Recording and Recall: This feature of the syringe electronics allows users to record and recall prior inflation information, making it ideal for procedure data tracking.7
Merit Sensor Systems: Pharmaceutical and Medical Solutions You Can Trust
Merit Sensor Systems’ AP Series pressure sensors are a top-tier option for a diverse suite of pharmaceutical and medical applications. The two configurations—gel-port with direct backside media contact and gel-port with dielectric gel—offer versatility and enhanced media compatibility while guaranteeing precision, robustness, and cost-effectiveness. Moreover, their compliance with stringent medical standards and integration into critical devices, such as Merit Medical’s Blue Diamond™ Digital Inflation Device, highlight their reliability and adaptability in demanding medical environments. To find out more about how these devices can be integrated into your workflow, contact a member of the Merit Sensor System’s team today.
References and Further Reading
-
- Casey, V., et al. (2011). Pressure Measurement at Biomedical Interfaces. Applied Biomedical Engineering. doi.org/10.5772/21855
- Merit Sensors. [Online] Applications. Available at: https://meritsensor.com/ (Accessed on 26 May 2024).
- Merit Sensor. Data Sheet: AP Series. Available at: https://meritsensor.com/assets/documents/pdf/AP-series.pdf (Accessed on 26 May 2024).
- Merit Sensor. [Online] AP Series. Available at: https://meritsensor.com/products/AP-Series/ (Accessed on 26 May 2024).
- Merit Medical. Blue Diamond™ Digital Inflation Device. Available at: https://cloud.merit.com/catalog/IFUs/407737001.pdf (Accessed on 02 July 2024).
- Merit Medical. [Online] Blue Diamond™ Digital Inflation Device. Available at: https://www.merit.com/product/blue-diamond-inflation-device/#documents_education (Accessed on 2 July 2024).
- Merit Medical. A Global Leader in Inflation. Available at: https://cloud.merit.com/catalog/Brochures/400899001.pdf (Accessed on 2 July 2024).